A-2
Immunity and Regeneration Integration Unit
7F
0714
Development of immunomodulation technology and quality control technology in cell therapy
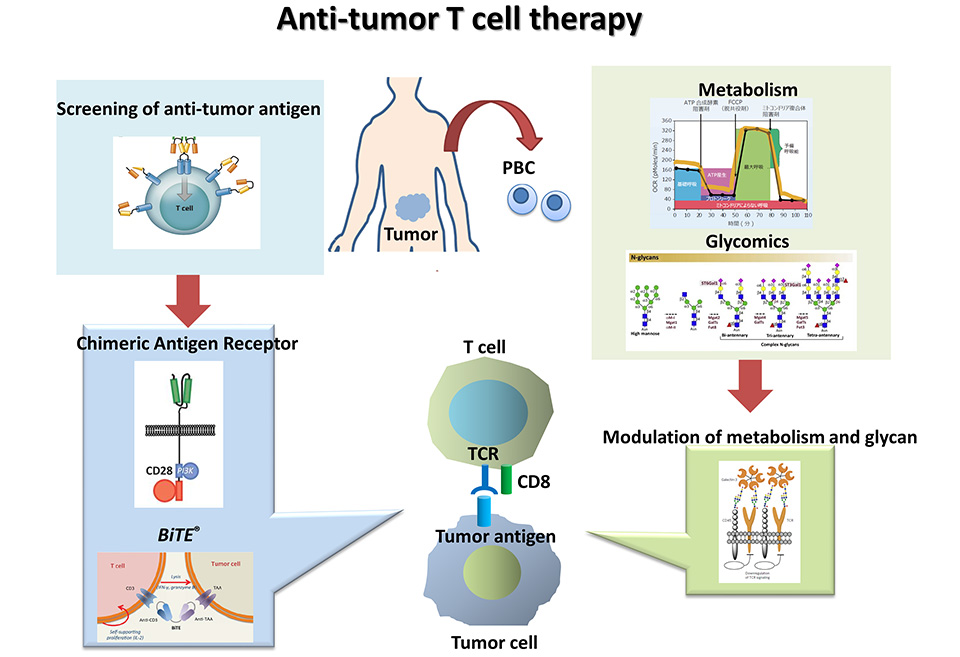
Cancer immunotherapy, represented by immune checkpoint inhibitors and CAR-T cells, has dramatically improved the long-term outcome of cancer treatment. On the other hand, only a small fraction of patients benefits from their clinical efficacy, and the development of combined immunotherapy and drug modification that can exert stronger and longer therapeutic effects is urgently needed.
This joint research team is working with Otsuka Pharmaceutical to develop a new treatment using what has been elucidated in basic research using preclinical murine models and in analysis using clinical specimens treated with immunotherapies. From the research on immunoregulation technology, we developed a new method for controlling the quality of immune cells by modification of glycosylation. Since this immuno-cell quality control technology can be applied for cancer immunotherapy, we are developing this technology to cell therapy in particular. Specifically, we will develop a novel cancer immunotherapy using activated T cells with newly established glycosylation modification. Furthermore, we will investigate the effector function and therapeutic duration by the induction of chemokines and neural guidance factors to regulate T-cell infiltration and maintain T-cell proliferation.
Responsible Department
Department of Immunology and Molecular Medicine
Research Partner
Otsuka Pharmaceutical Co., Ltd.
Project Members
Principal Investigator

Associate Professor
Department of Respiratory Medicine and Clinical Immunology
Members
Specially Appointed Assistant Professor
Department of Immunology and Molecular Medicine
Specially Appointed Associate Professor
Department of Immunology and Molecular Medicine
Assistant Professor
Department of Respiratory Medicine and Clinical Immunology
Otsuka Pharmaceutical Co., Ltd.
Otsuka Pharmaceutical Co., Ltd.
Otsuka Pharmaceutical Co., Ltd.
Links
Otsuka Pharmaceutical Co., Ltd.
https://www.otsuka.co.jp/en/




















