A-4
Immunopharmaceutical Development Unit
8F
0803, 0804
Clinical research for regulatory factors
in tumor immunology
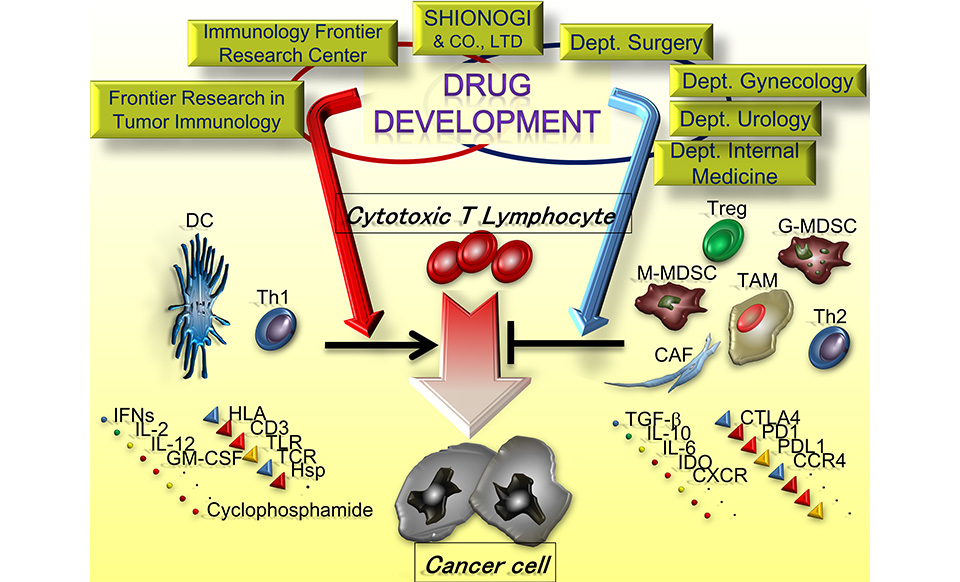
Emerging interest in new cancer immunotherapy through the regulation of immunosuppressive mechanisms has attracted worldwide attention because of the remarkable clinical effects of immune checkpoint inhibitors. With the full support of Professor Sakaguchi (IFReC), who discovered regulatory T cells (Tregs), our laboratory explores mechanisms to regulate various immunosuppressive cells, especially Tregs, to chase up new drug seeds for clinical application of cancer immunotherapy in collaboration with Shionogi & Co., Ltd.
To conduct clinically oriented research, especially detailed analysis in tumor micro-environment, we have established close cooperation with the departments of gastroenterological surgery, gynecology, urology, dermatology, breast surgery, head and neck surgery, respiratory surgery, and respiratory medicine. Through this broad collaboration, we have newly identified and patented “CCR8”, uniquely expressed on tumor-infiltrating Tregs. Furthermore, we have produced an antibody drug targeting CCR8, and a clinical trial has been initiated in 2022.
Responsible Department
Department of Clinical Research
in Tumor Immunology
Research Partner
Shionogi & Co., Ltd.
Project Members
Principal Investigator

Specially Appointed Associate Professor
Department of Clinical Research
in Tumor Immunology
Links
Department of Clinical Research in Tumor Immunology, The University of Osaka
http://www.climm.med.osaka-u.ac.jp/
Shionogi & Co., Ltd.
http://www.shionogi.co.jp/en/




















