A-22
Immunopharmaceutical Development Unit
9F
0912A, 0914B
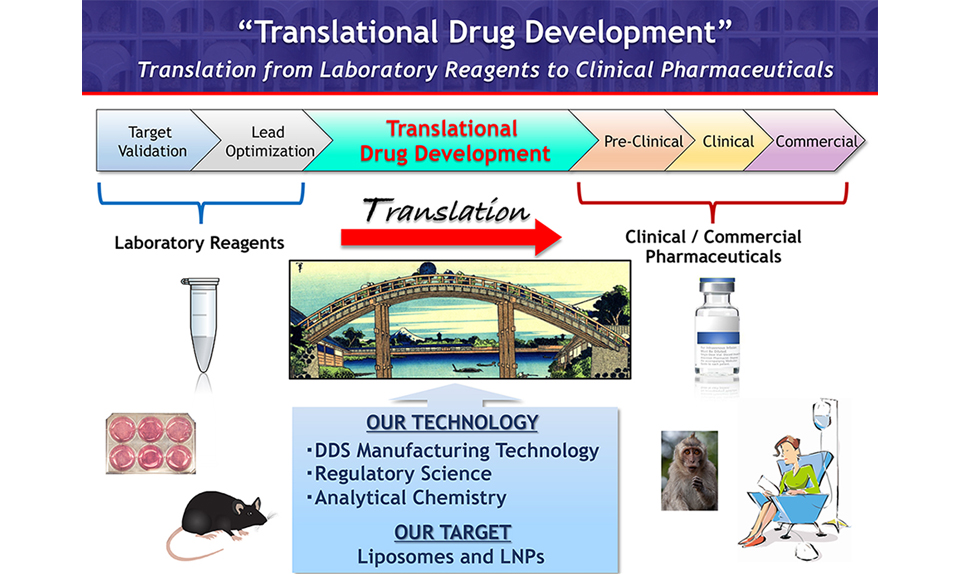
Development of cGMP manufacturing process for novel lipid-based nano-medicine by taking advantage of the in-line manufacturing platform technology
The aim of our department is the development of current good manufacturing practice (cGMP) manufacturing processes for novel lipid-based nano-formulations, including liposomes and lipid nano-particles (LNPs), targeting immunocompetent cells for the treatment of cancer and transplant rejection. Our innovative and patented in-line manufacturing technologies, SOLID and SQUID, have proven to be promising for cGMP production of liposomal investigational new drugs. Our technologies will accelerate the “Translational drug development” in which laboratory reagents are re-developed to clinical and commercial pharmaceuticals, and therefore lead to prompt approval of new drugs.
Responsible Departments
Department of DDS Pharmaceutical Development
Research Partner
Shionogi Pharma Co., Ltd.
Project Members
Principal Investigator

Specially Appointed Professor
Department of DDS Pharmaceutical Development
Links
Shionogi Pharma Co., Ltd.
https://www.shionogi-ph.co.jp/en/
Contact:
info(at)dds.med.osaka-u.ac.jp(※Please replace (at) with @.)




















