A-24
Novel Integrated Area Unit
9F
0914
Building new clinical evidence by integrating clinical trial data and real world data (RWD)
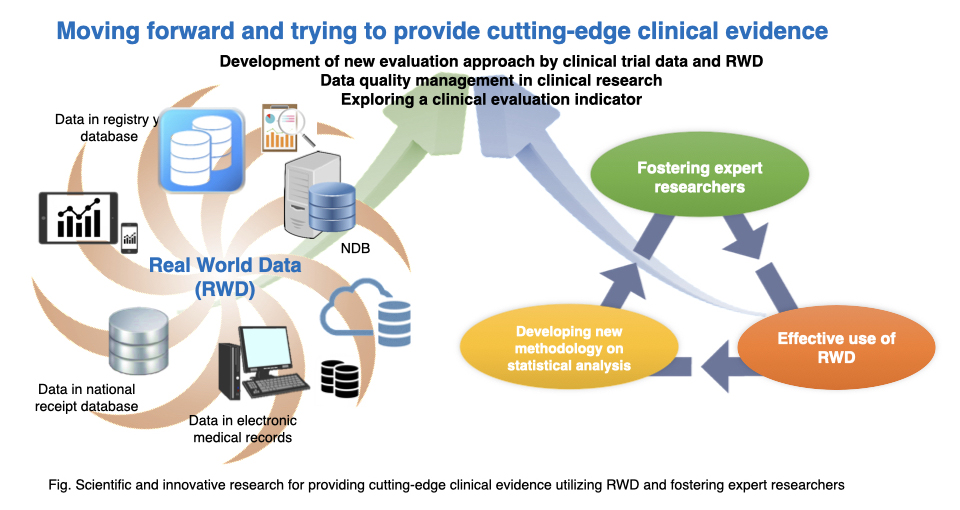
RWD such as data from electronic medical record, national receipt database or patient registry are really valuable information source which can indicate actual health care environment and that is receiving a lot of attention recently because, we are expecting that effective use of RWD can optimize clinical development strategies, build clinical evidence and highly effective pharmacovigilance system and finally, take a part of enhancing clinical evaluation efficiency.
However, we also recognize there are a lot of challenges in terms of appropriate use of RWD and interpretation of research results obtained from RWD and especially, in the regulatory environment, we definitely need to overcome a lot of difficulties such as data quality and data collection methodologies of RWD.
Our department mission is to contribute to health care improvement and the realization of healthy society with overcoming following diverse difficulties in relation to effective use of RWD in collaboration with department of integrated medicine biomedical statistics and data coordinating center as well.
・Investigating methodology of collection and management of RWD focusing on data quality
・Developing new evaluation methodology for medicinal products as well as medical devices by integrating clinical trial data and RWD
・Exploring new clinical evaluation indicator with utilizing RWD
In order to obtain high quality evidence from clinical research, biostatistical literacy such as “designing scientific protocol”, “data management for ensuring conformity of data” and “Statistical analysis and appropriate interpretation of study results” is really important.
In addition to above, we aspire to foster expert researchers with expertise in epidemiology, statistics, bioinformatics and medical information which are foundation of medical data science.
Responsible Departments
Department of Biostatistics and Data Science
Research Partner
SHIONOGI & Co., Ltd.
Project Members
Principal Investigator

Specially Appointed Professor
Department of Biostatistics and Data Science
Members
Specially Appointed Associate Professor
(Lecturer)
Department of Biostatistics and Data Science
Specially Appointed Assistant Professor
Department of Biostatistics and Data Science
Specially Appointed Professor
The University of Osaka Hospital, Department of Medical Innovation, Data Coordinating Center
Associate Professor(Lecturer)
Hiroshima City University
Joint Research Collaborator / Guest Professor
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Joint Research Collaborator
SHIONOGI & Co., Ltd.
Links
SHIONOGI & Co., Ltd.
https://www.shionogi.com/global/en/




















